
Snap & Share Contest
Take a moment to capture a photo that reflects the spirit of the conference or highlights a specific theme. Post it on LinkedIn and tag World BI. The photo with the most likes wins a Surprise Gift! Ready, set, SNAP!

Healing Begins with Top-Quality Supplies
About CTS 2026
The Clinical Trial Supply Forum is a premier B2B platform uniting industry leaders, innovators, and stakeholders across the clinical supply chain. This two-day event is designed to address the complexities of clinical trial logistics, discuss emerging technologies, and share strategic solutions that drive efficiency, transparency, and compliance in global clinical supply operations.
Learn More
We champion agile, tech-driven clinical supply that enhances patient access, sustainability, and speed. Our goal is to equip supply leaders with tools to cut risk, boost resilience, and accelerate therapy delivery.
We see clinical supply chains that are smart, digital, and patient-first—powered by real-time tracking, decentralized delivery, and sustainable practices. CTS 2026 is where strategy meets innovation in supply excellence.
in supply chain planning, patient-centric trials, and risk mitigation.
on temperature-controlled logistics, direct-to-patient models, and IRT integration.
on managing decentralized trials and overcoming import/export challenges.
with supply chain leaders, clinical trial sponsors, CROs, and technology vendors.

External Manufacturing Organization - Consumer Health LatAm ; Community Board Chair at CSCMP

Director of Quality

Senior Manager, Clinical Research Sourcing Strategist

Sr. Director, Clinical Supply Chain

Associate Professor of Supply Chain and Information Systems Management

Director, Global Regulatory Affairs Devices

Sr. Director, Clinical Supply Chain

Associate Director, Patient Centricity
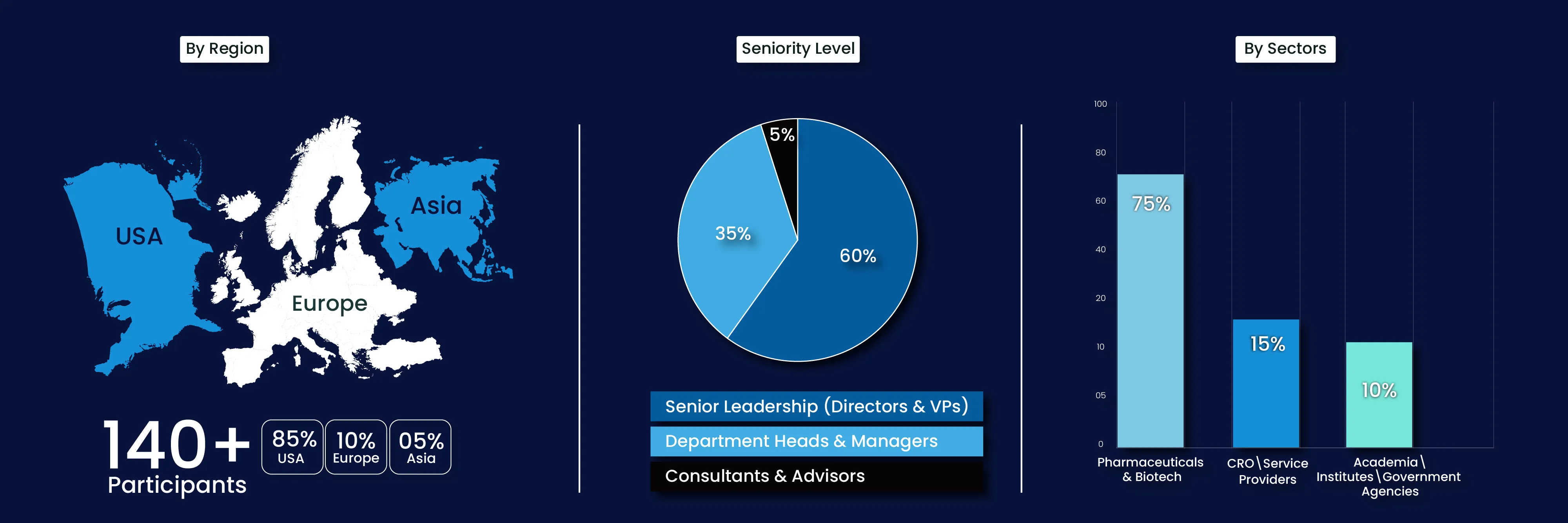
Meet the Global Community of Health Innovators






















































Take a moment to capture a photo that reflects the spirit of the conference or highlights a specific theme. Post it on LinkedIn and tag World BI. The photo with the most likes wins a Surprise Gift! Ready, set, SNAP!

As part of the Scavenger Hunt, connect with five new attendees by introducing yourself and exchanging business cards. Learn about their expertise, and once you've collected five unique cards, note their names or take a photo.

Enjoy light refreshments in a casual setting, perfect for continuing conversations, building new relationships, and networking after a day of insightful sessions.

We want to hear from you. Share your perspective in the comments and let's engage in a thoughtful discussion to explore the challenges and solutions. Question will be announced soon!!

Speed networking is a fast-paced, organized activity that helps participants make connections with a lot of people quickly and develop business ties.
Held during the lively Fleet Week, the conference offers a unique and vibrant festive experience. Taking place annually in October, San Francisco Fleet Week 2025 is scheduled for October 5–13, and we’re proud to be part of the early coordination efforts to align our event with this iconic citywide celebration.
Hosted in the legendary city of San Francisco, the conference is set against a backdrop of world-renowned landmarks such as the Golden Gate Bridge.