
Clinical Trial Supply Forum
The Premier Event for Leaders Shaping the Future of Fast-Moving, Global Clinical Trial Supply
A strong clinical trial supply chain ensures that the right investigational product reaches the right patient, at the right time and in the right amount. Today, supply chains face challenges like unpredictable demand, complex global distribution, temperature-sensitive logistics, regulatory rules, and limited real-time visibility.
The Clinical Trial Supply Forum 2026 – US Edition is designed to tackle these challenges. Over two days, the forum will cover risk management, logistics, packaging, labeling, and storage, giving senior leaders practical strategies to make clinical trial supply chains faster, smarter, and more reliable.
What We'll Explore
Decentralized & Hybrid Trials
Adapting supply strategies for remote and hybrid study designs.
Patient Centric Logistics (DTP)
Enhancing access and engagement through direct-to-patient delivery.
Digital & Real-Time Visibility (IRT/RTSM)
Leveraging IRT/RTSM systems and AI for smarter forecasting and inventory management.
Regulatory Compliance & Comparator Sourcing
Ensuring consistency and compliance across countries.
Sustainable & Resilient Supply Networks
Reducing environmental impact while mitigating risks.
Cross Functional Collaboration
Aligning clinical, manufacturing, and logistics teams for supply readiness.
Connect with the Global Leaders Driving Clinical Trial Supply Excellence
Meet the Global Community of Health Innovators
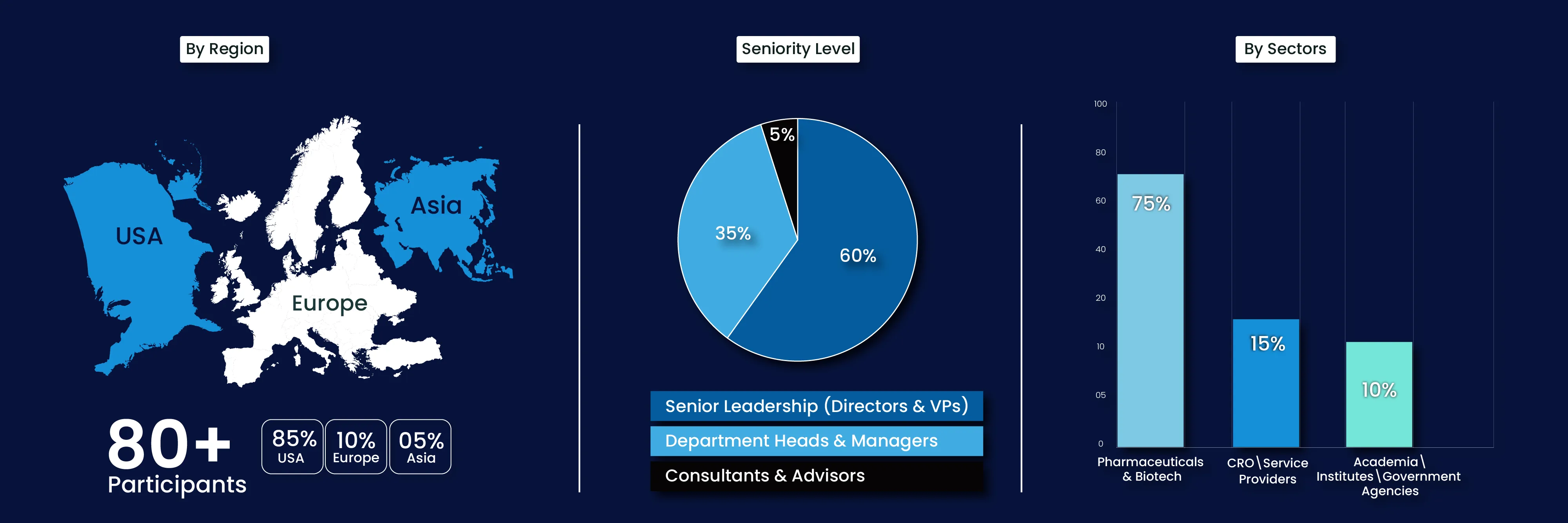
Our Past Attendees Have Been From…

























































